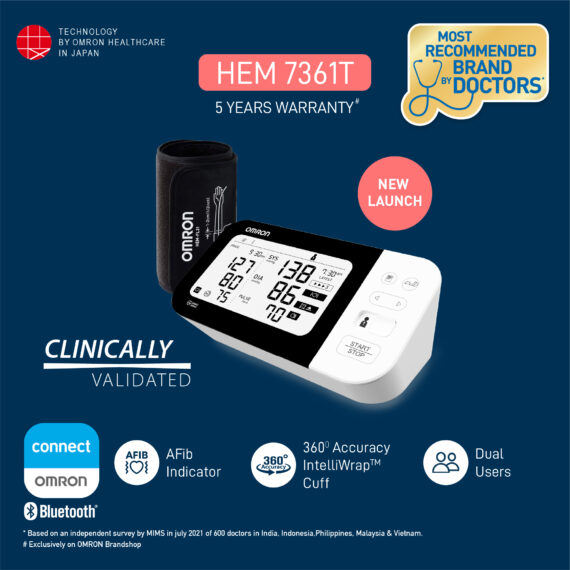
Omron HEM 7361T with bluetooth®, AFIB Indicator, IntelliWrap™ Cuff & Dual User Feature
Offer Price
₹5,978.00₹7,290.00
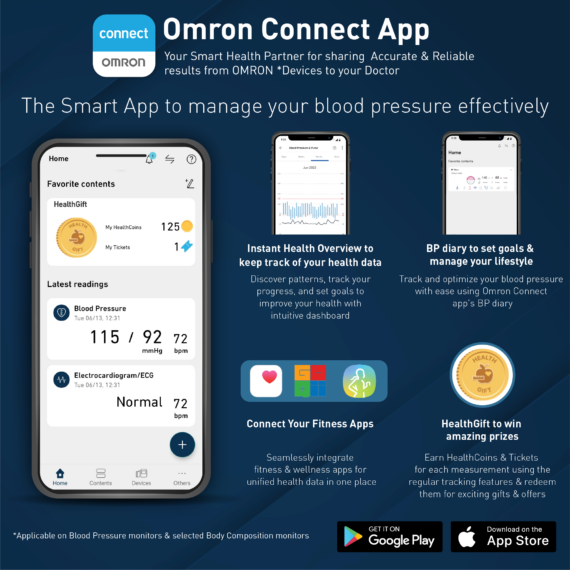
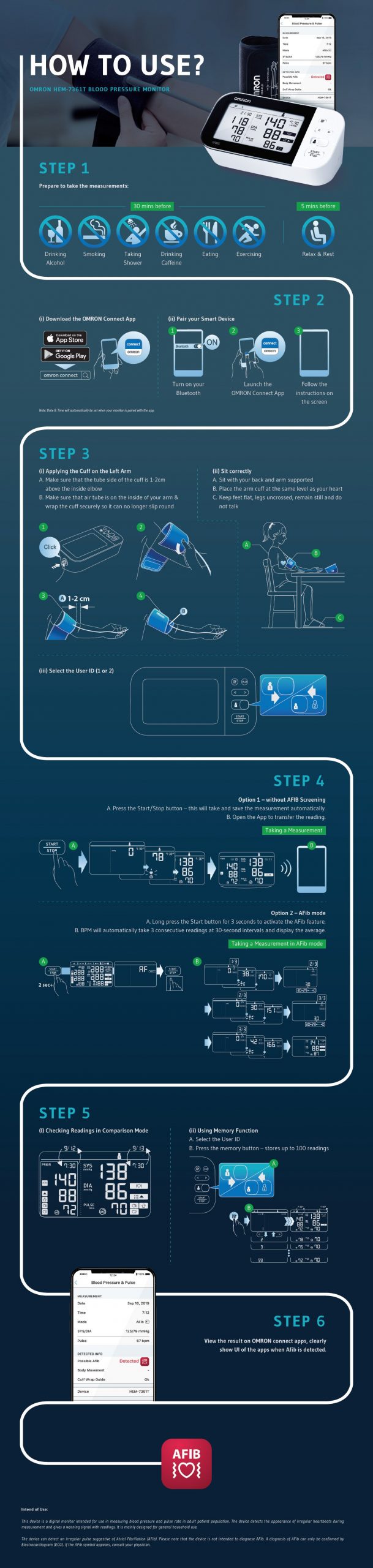
Professional Grade Omron HEM 7361 T BP monitor is your comprehensive heart health management partner as beside measuring BP it also allows you to diagnose AFib and risk of strokes in one seating with the help of its Dual Check feature. Designed to take care of the heart health of your family members, the dual user function allows syncing of measurements to smartphones for up to 2 users.
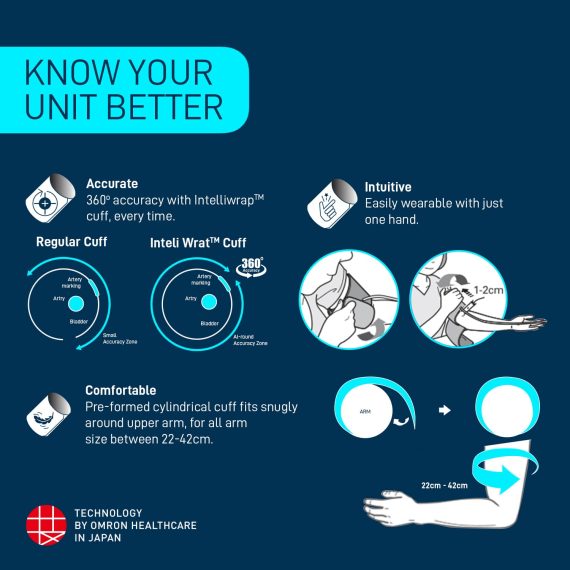
Intelli Wrap Fit Cuff (22-42 cm)
Net Quantity : 1 automatic blood pressure monitor set (1 main unit, 1 intelli wraptm cuff, 1 storage case, 4 aa batteries, 2 instruction manual, 1 omron connect setup leaflet, 1 warranty card)
Manufactured By : Omron healthcare manufacturing vietnam co., ltd. no. 28, vsip ii, street 2, vietnam – singapore industrial park ii binh duong industry – services – urban complex hoa phu ward, thu dau mot city, binh duong province vietnam services – urban complex hoa phu ward, thu dau mot city, binh duong province vietnam
Imported And Marketed By : Omron healthcare india pvt. ltd.
Reg. Office : 6th floor, b – block, sewa tower, plot no-19, sector-18, udyog vihar, maruti industrial complex, gurugram, haryana (india) – 122008
Import License No : IMP/MD/2021/000052
Lm Model Approval No : IND/09/20/25
Country of Origin : Made in Vietnam
For any complaints or feedback contact customer care executive at imported and marketed by address above or tel : 18 00 41 90 492 or write us at e-mail address : callcentre@omron.com
Website : www.omronhealthcare-ap.com/in
In stock
Check estimated delivery date
Description
Additional information
| Weight | 0.34 kg |
|---|---|
| Dimensions | 12 × 12 × 20 cm |
Reviews(14)
Only logged in customers who have purchased this product may leave a review.



































Dominic Dsouza
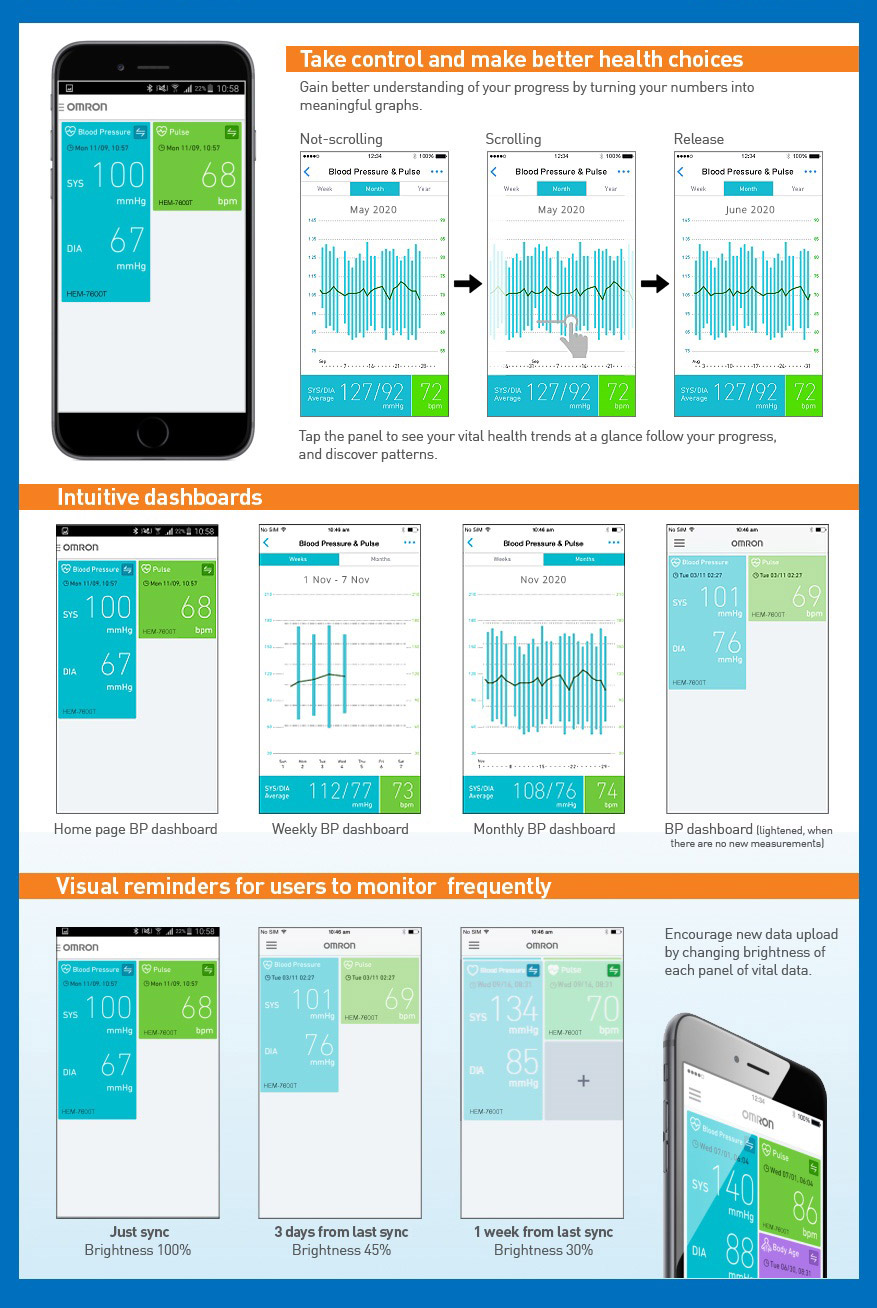
HEM-7361T is a very good BP monitoring device for 2 users, i am currently using it for over a month and i am very happy with it. All readings are synced with the omron connect app using bluetooth. Omron connect app is great for viewing all historical BP measurements in a graph visualization. A great buy i would say!
Muhammad Imran Khan
Perfect Blood Pressure Monitor , comfortable to use 360° intelli Wrap. high quality product ,Love each bit of it and the way its pack and delivered.wonderful work team Omron …keep it up
Rgds Muhammad Imran Khan
Ashok Kumar
(verified owner)This is the best product to have. I have been using it for 15 days, and it is helping me track my blood pressure and manage it. Simply an awesome product and a must have at home.
Harshal Purohit
I have been using some other monitors for years, however, for the first time I have been feeling that the readings are quite accurate. Besides, the usage of instrument, the connectivity of app, the other features of app including health gifts tempt me to measure the blood pressure religiously; without fail. Thank you Omron for the soothing experience.
satyamili
I am very happy with this purchase of Automatic Blood Pressure Monitor HEM-7361T and it gives quire accurate readings . More importantly my father 84+ yrs is able to operate it very easily . Thank you Omron .
arunsharma9168
Excellent blood pressure measuring device.
Aparna Yadav
(verified owner)It is a user-friendly machine with many features. Worth buying. Thanks.
roshan0388
(verified owner)Very accurate and professional. Mobile app is motivating
swaathi777
Hi sir, recently i brought omron bp machine with normal size cuff but i need extra large cuff in same omron brand,where do i get. Pls rply. Thank in advance
Rohit Agarwal
(verified owner)Bought this product a month back.
Pros-
-Delivered very quickly in a very nice packing.
-As part of promotion got Omron pedometer free.
-Overall device built quality is best – machine as well as the cuff, even the storage bag.
-Takes the ready quickly and stores without any error.
-Very easy to use. Quick reading can be taken just by a button press.
-Mobile app is also easy to use and stores the readings in a way which is easy to look at.
-Got 2 years extra warranty as I bought through Omron brand shop.
Cons-
-There is little variation in readings (taken multiple times). I think this is how such product generally works.
-Connecting mobile through Bluetooth is sometimes challenging. Need to read instructions very carefully, else it will not connect. It would have been better if device automatically connects to mobile once it is switched on (the way Bluetooth headphones work).
-User manual could have been more explanatory. Most of the steps are explained using images. Although this is good approach but sometimes some smaller steps are tough to understand.
HIMADRI CHATTERJEE
(verified owner)Feedback:
Although I am not a Medical practioner, but immensely satisfied with the product. Found the product user friendly/easy to operate.
Was able to record the readings through the Omron mobile app and share a pdf report with my doctor.
The readings were close and I want to believe that it is accurate. Trust played a major role here as I am not a specialist to comment on its accuracy.
Area of opportunitiy:
Although the graphical presentation of the product in the user manual is self explanatory but important details about the data getting captured along with details on “AFB” indicator should have been elaborated. It would have helped to bridge our understanding gaps and realize when to raise an alarm, or avoid raising false alarm. The intent here is to realize that it would also be used by a “Common Man” not by Medical practioners only.
Sanjit Kumar Paul
(verified owner)I am a para medico. In our hospital we specially use Omron product because we can believe this brand. This product, HEM 7361t, I purchased for my father. He is a stroke patient. So, we need to check on daily basis. Since 3 to 4 months, it is working fine. Best thing is AFIB indicator. I’ll also submit review one year after using.
I have two suggestions.
1. If company add option for charger (yes/no) with product and its price also, it could be better. Because, if I want to purchase the charger, I have to wonder market again. If customer willing to add they will pay extra otherwise only machine can purchase. It’s my point of view.
2. At the time of delivery I faced some problem with courier. I also talked to customer service and told my problem. So, company should tie-up with some good courier company. If y problem is single, then its ok, no need to think about it, otherwise should think.
Thanks & Regards
Sanjit Kumar Paul
sanjitkumarplexcel@gmail.com
ArunM
(verified owner)Worst product….Readings are not at all accurate…. Shows reading way less than actual number. And moreover Poor response from customer care.
Rakesh Alhan
(verified owner)Not very much sure on the accuracy, but giving only 1 star, because of I’m unable to connect the device with my mobile OnePlus 5T, which is already listed in compatible devices, even after 20 days, even after multiple follow ups. Technical team is still clueless on the resolution, and asking me to return the product. Simply don’t have any productive mindset.
P.S. need to purchase the adopter seperately
senthilnayagam
(verified owner)The device is easy to setup and use. The reading logs and graphical view is nice in the App but the App itself could be improved further (there is no Home or back button).