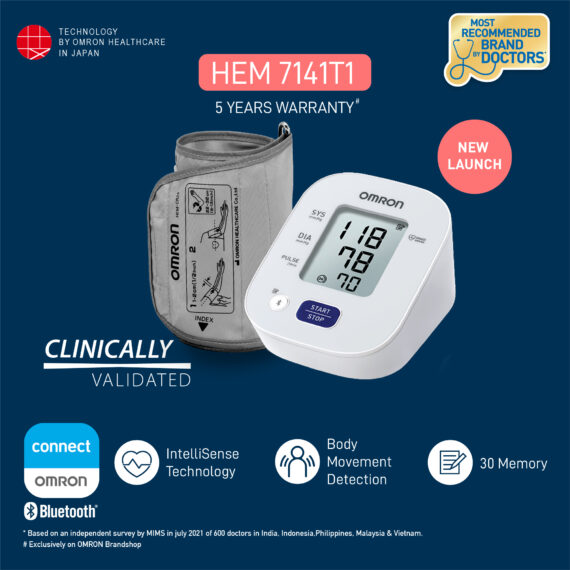
Omron HEM 7141T1 Bluetooth Blood Pressure Monitor With Body Movement Detection & Cuff Wrapping Guide Technology
Offer Price
₹2,344.00₹2,900.00
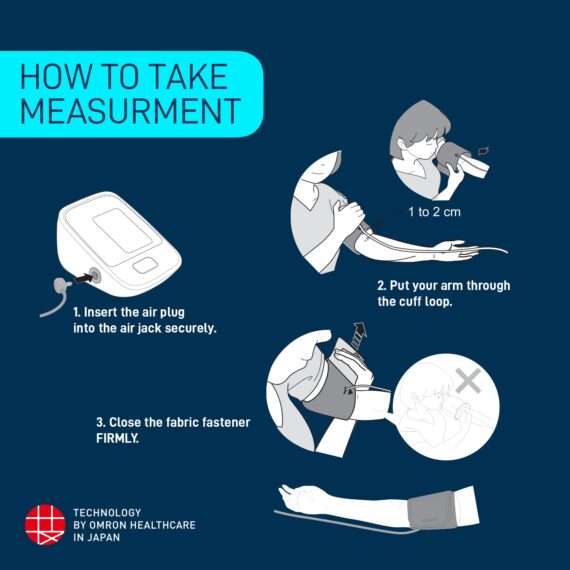
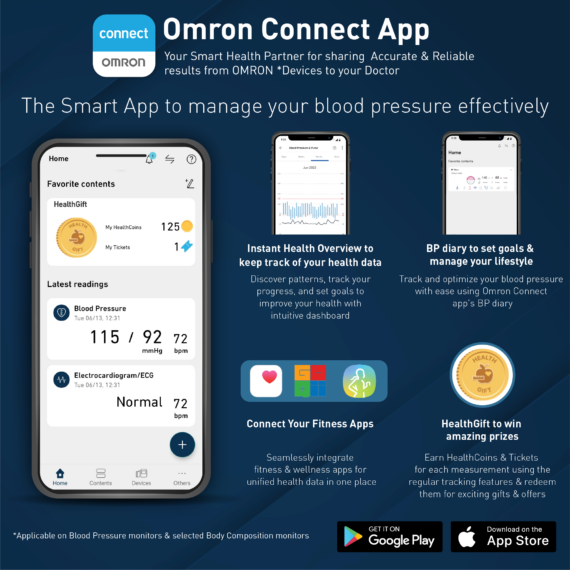
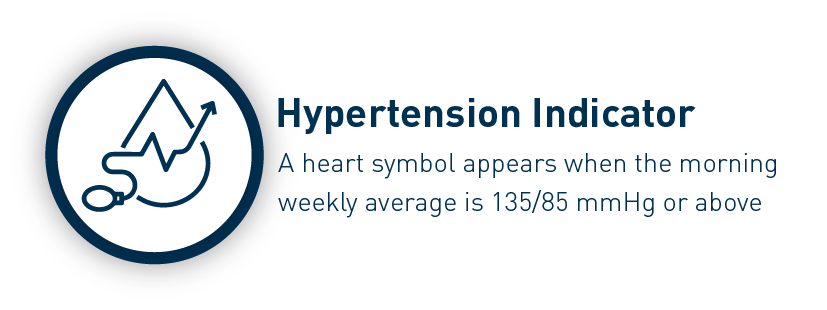
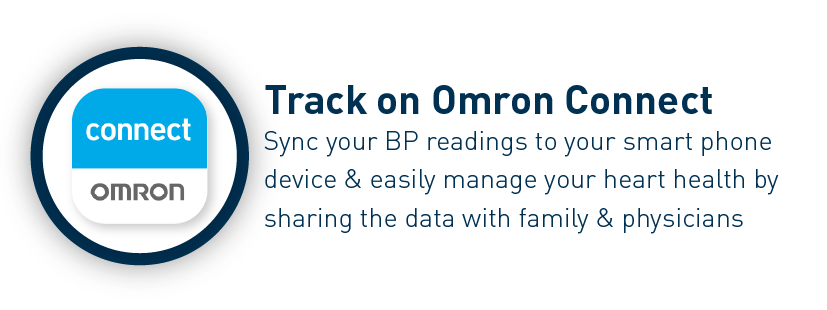
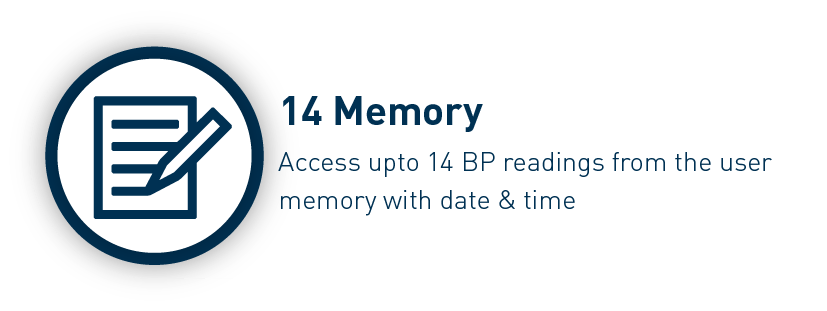
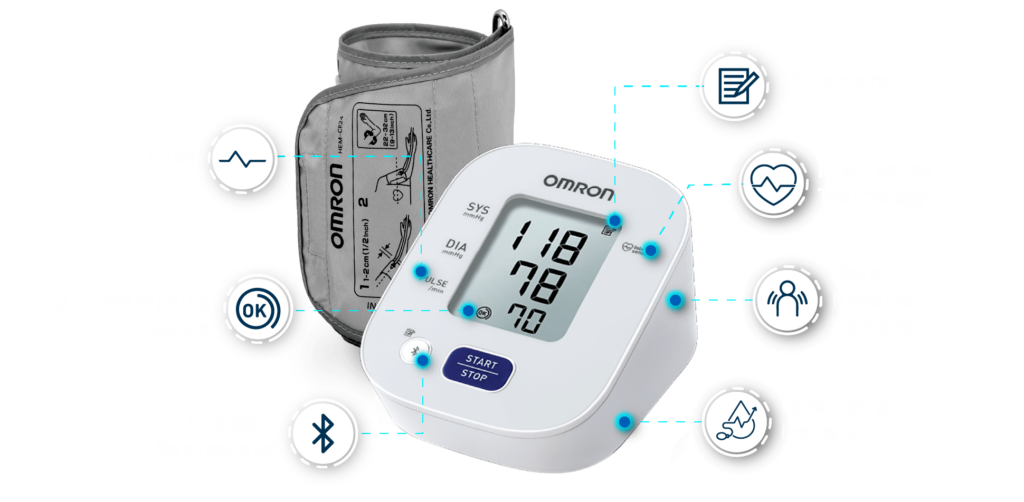
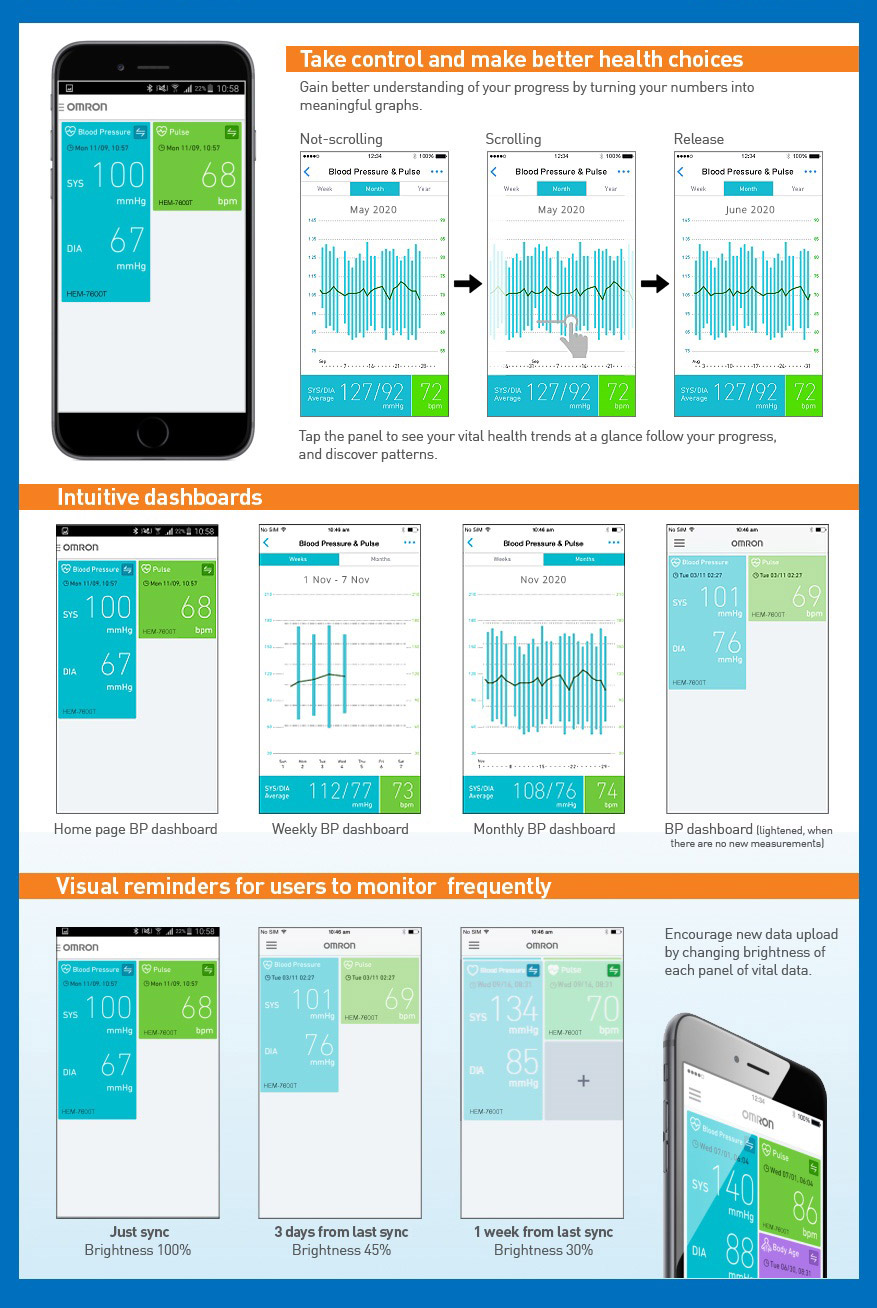
The Omron HEM-7141T1 digital blood pressure monitor is clinically validated for accuracy and reliability. It features a cuff-wrapping guide to assist you in correctly positioning and wrapping the cuff around your arm. The cuff wrapping guide ensures that you achieve the correct fit and positioning, optimizing the accuracy of your readings. The innovative IntelliSense™ technology enables the device to automatically personalize cuff inflation according to the size of your arm. By tailoring the inflation to your specific arm size, it ensures that the pressure applied by the cuff is suitable for an accurate reading while maximizing your comfort during the measurement process. Additionally, the HEM-7141T1 is equipped with body movement detection. This feature detects excessive movement during measurements and provides an alert if motion interference may have affected the accuracy of the reading. The blood pressure monitor also includes a hypertension indicator, which serves as an added safety feature. If your blood pressure reading falls within the hypertensive range, the device will promptly alert you. This feature allows you to be aware of any potential high blood pressure readings, enabling you to take necessary precautions and seek appropriate medical advice if needed. With the added Bluetooth connectivity, the HEM-7141T1 blood pressure monitor allows you to effortlessly sync your measurements with compatible devices. The Bluetooth functionality enhances convenience and enables seamless integration into your digital health ecosystem. The Omron HEM-7141T1 blood pressure monitor is equipped with a memory function that allows you to store and review your previous blood pressure readings. With its 14-memory storage capacity, you can track your blood pressure measurements over time and monitor any changes or trends.
Net Quantity : 1 automatic blood pressure monitor set (1 main unit, 1 arm cuff, 4 aa batteries, 1 instruction manual)
Manufactured By : omron healthcare manufacturing vietnam co., ltd. no. 28, vsip ii, street 2, vietnam – singapore industrial park ii binh duong industry – services – urban complex hoa phu ward, thu dau mot city, binh duong province vietnam
Imported And Marketed By : Omron healthcare india pvt. ltd.
Reg. Office : 6th floor, b – block, sewa tower, plot no-19, sector-18, udyog vihar, maruti industrial complex, gurugram, haryana (india) – 122008
Import License No : IMP/MD/2021/000052
Lm Model Approval No : IND/09/21/812
Country of Origin : Vietnam
For any complaints or feedback contact customer care executive at imported and marketed by address above or tel : 18 00 41 90 492 or write us at e-mail address : callcentre@omron.com
Website : www.omronhealthcare-ap.com/in
Out of stock
Description
Additional information
| Weight | 0.7 kg |
|---|---|
| Dimensions | 14 × 15 × 18 cm |
Only logged in customers who have purchased this product may leave a review.





















Reviews
There are no reviews yet.